What is ductal carcinoma in situ (DCIS)?
Ductal carcinoma in situ (DCIS) is a non-invasive breast cancer. It’s stage 0 breast cancer.
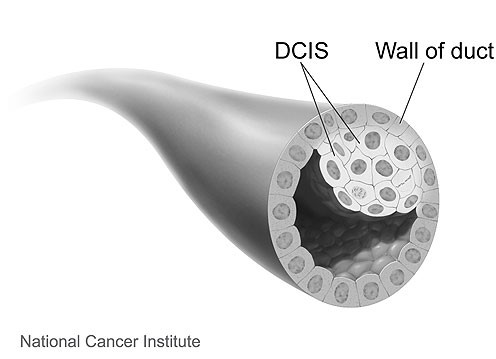
“Ductal” means “related to the milk ducts”. The milk ducts are the canals that carry milk from the lobules to the nipple openings during breastfeeding. “In situ” means “in place”. With DCIS, the abnormal cells haven’t broken through the walls of the milk ducts and haven’t spread to nearby breast tissue.
Without treatment, DCIS cells could progress to invasive cancer over time. So, you may hear the terms “pre-invasive” or “pre-cancerous” to describe DCIS.
DCIS in the United States
About 15 percent of breast cancers in the U.S. are DCIS [1]. More than 48,000 new cases of DCIS are diagnosed in the U.S. each year [2].
Most cases of DCIS are in women over 50 [1]. In the U.S., most often, DCIS is first detected on a screening mammogram [3].
Learn about diagnosis of DCIS.
Time trends of new cases of DCIS
Rates of new cases of DCIS in the U.S. seem to be decreasing slightly over time. From 2013-2017, rates decreased by about one percent each year [4].
Prognosis
With treatment, prognosis (chance of survival) for DCIS is usually excellent.
DCIS diagnosed with invasive breast cancer
Sometimes, the tissue removed during a biopsy or breast surgery shows both DCIS and invasive breast cancer. If DCIS is diagnosed with invasive breast cancer, treatment and prognosis are based on the invasive breast cancer, not the DCIS.
Learn more about treatment for early invasive breast cancer.
Treatment for DCIS
The recommended treatment for DCIS is surgery, with or without radiation therapy [5]. After surgery and radiation therapy, some people take hormone therapy [5].
Chemotherapy is not used to treat DCIS.
Find a list of questions to ask a doctor about treatment for DCIS.
Thelma Brown, a Komen advocate who has DCIS advises, “A diagnosis of any stage of breast cancer can be very frightening. Often, the first instinct is to act quickly. There is not a one size fits all approach to breast cancer, and DCIS is no different. Therefore, it is important to slow down and take the time to learn about DCIS and your treatment options. This will enable you to be an active member of your health care team and share in the decision making. Also, taking care of your mental and emotional health is vital. If needed, do not hesitate to get support from family, friends, survivors or counselors. The goal is a full and healthy life, in spite of breast cancer.”
Surgery
Surgery for DCIS removes the abnormal tissue from the breast. It can be lumpectomy or mastectomy.
If there’s little spread of DCIS within the breast, a choice can be made between lumpectomy and mastectomy. If DCIS affects a large part of the breast, a total (simple) mastectomy will be done.
Overall survival is the same for women with DCIS who have lumpectomy (with or without radiation therapy) and those who have mastectomy [5].
Lumpectomy
With lumpectomy, the surgeon only removes the abnormal tissue in the breast. The rest of the breast is left intact. Lymph nodes aren’t usually removed.
Mastectomy
With total mastectomy, the surgeon removes the entire breast, but no other tissue.
Sometimes, a sentinel node biopsy, which removes 1-5 lymph nodes in the underarm area, is done at the same time as a mastectomy for DCIS. A sentinel node biopsy is done just in case invasive breast cancer is found in the breast tissue removed during surgery.
Learn more about surgery for DCIS.
Radiation therapy
After lumpectomy
Lumpectomy for DCIS is usually followed by whole breast radiation therapy.
Overall survival is the same for women with DCIS who have lumpectomy with radiation therapy and those who have lumpectomy without radiation therapy [5-7].
Although whole breast radiation therapy after lumpectomy doesn’t impact survival, it lowers the risk of [5-12]:
- DCIS recurrence (a return of DCIS) in the treated breast
- Invasive breast cancer in the treated breast
Some women with DCIS may have the option of partial breast radiation therapy or skipping radiation therapy altogether [5]. These women must have [5,12-13]:
- Small, low grade DCIS (the DCIS cells look similar to normal cells)
and - Clean surgical margins (the area of tissue surrounding the DCIS removed during surgery contains no DCIS cells)
Find a summary of research studies on lumpectomy plus radiation therapy in the treatment of DCIS.
After mastectomy
Radiation therapy is rarely given to women treated with mastectomy for DCIS. Learn more about radiation therapy for DCIS.
Hormone therapy
Some DCIS tumors are hormone receptor-positive (estrogen receptor-positive/progesterone receptor-positive). This means the DCIS cells express (have a lot of) hormone receptors.
After lumpectomy
Women who are treated with lumpectomy for hormone receptor-positive DCIS may take a hormone therapy pill (tamoxifen or an aromatase inhibitor) [5]. Hormone therapy can lower the risk of [5,9,14-18]:
- DCIS recurrence
- Invasive breast cancer
These risks are lowered in both the treated breast and the opposite breast.
Find a summary of research studies on tamoxifen in the treatment of DCIS.
After mastectomy
Hormone therapy isn’t recommended for women who have a mastectomy for DCIS [5]. These women have an excellent prognosis with a very low risk of DCIS recurrence or developing breast cancer in the opposite breast.
Learn more about hormone therapy for DCIS.
Follow-up care after treatment for DCIS
After treatment for DCIS, recommended follow-up care includes regular mammograms and clinical breast exams (physical exams) [5].
Learn more about medical care after treatment.
Risk of developing invasive breast cancer after treatment for DCIS
After treatment for DCIS, there’s a small risk of invasive breast cancer, as well as DCIS recurrence [5].
These risks are higher with lumpectomy plus radiation therapy than with mastectomy [2]. Overall survival is the same after either treatment [2].
Higher grade DCIS (the DCIS cells look abnormal) appears more likely than lower grade DCIS (the DCIS cells look similar to normal cells) to progress to invasive cancer after treatment [19].
Ten years after DCIS diagnosis, studies show about 2-6 percent of women have a DCIS recurrence in the opposite breast or an invasive breast cancer in the opposite breast [20-23].
Kornelia Polyak, MD, PhD, a Komen Scholar and Professor of Medicine, Dana-Farber Cancer Institute, Harvard Medical School, in Boston, MA shared her thoughts on DCIS and recurrence. According to Dr. Polyak, “Even though DCIS by itself is not a life-threatening disease, understanding why some patients with DCIS develop invasive breast cancer while others do not would help our understanding of drivers of tumor progression and the design of more effective therapies.”
Does every case of DCIS need treatment?
Although the exact percentage is not known, it’s estimated about 20-50 percent of DCIS cases progress to invasive breast cancer if left untreated [24-28].
Doctors can’t predict which cases of DCIS will progress and which won’t so that’s why almost all cases of DCIS are treated.
DCIS and over-treatment
Some cases of DCIS are considered over-treated because they will never progress to invasive breast cancer. Even without treatment, the DCIS would never have caused symptoms or problems. This means some people are over-treated for DCIS. They get little benefit from treatment, beyond peace of mind.
Researchers are studying ways to predict which cases of DCIS will progress to invasive breast cancer. This would allow some people at low risk to avoid treatments they don’t need.
Clinical trials for DCIS
After discussing the benefits and risks with your doctor, we encourage you to join a clinical trial if there’s one right for you.
If you or a loved one needs information or resources about clinical trials, call Susan G. Komen’s® Breast Cancer Clinical Trial Information Helpline at 1-877 GO KOMEN (1-877- 465- 6636) or email clinicaltrialinfo@komen.org.
BreastCancerTrials.org in collaboration with Susan G. Komen® offers a custom matching service to help find clinical trials that fit your health needs.
Summary
DCIS is a non-invasive breast cancer, but it may progress to invasive breast cancer over time.
Not all DCIS will progress to invasive breast cancer, but doctors can’t tell which DCIS will progress and which won’t. So, almost all cases of DCIS are treated.
Treatment for DCIS includes some combination of surgery, radiation therapy and/or hormone therapy. With treatment, the prognosis for DCIS is usually excellent.
Unfortunately, some cases of DCIS are over-treated. Researchers are looking at ways to predict which cases of DCIS are the most likely and the least likely to progress to invasive breast cancer. This research may help some people with DCIS avoid over-treatment.
Komen resources
If you have questions or concerns about DCIS and want to talk to someone, call our Breast Care Helpline at 1-877 GO KOMEN (1-877-465-6636). Calls to our helpline are answered by a trained and caring staff member Monday – Thursday 9 a.m. EST – 7 p.m. EST and Friday 9:00 a.m. – 6:00 p.m. EST. You can also email the helpline at helpline@komen.org.
For more information about DCIS on komen.org, please visit:
- Treatment for DCIS
- Questions for your doctor about DCIS
- Emerging areas in the treatment of DCIS
- Facts for Life: Ductal carcinoma in situ
- Facts for Life: What is breast cancer?
- Komen-funded research
References
- American Cancer Society. Breast Cancer Facts and Figures 2019-2020. Atlanta, GA: American Cancer Society, 2019.
- American Cancer Society. Cancer Facts and Figures 2020. Atlanta, GA: American Cancer Society, 2020.
- National Cancer Institute. Breast cancer screening (PDQ®)–health professional version. https://www.cancer.gov/types/breast/hp/breast-screening-pdq – _66_toc, 2020.
- Howlader N, Noone AM, Krapcho M, et al. (editors). Cancer Statistics Review, 1975-2017. Table 4.4: Trends in SEER incidence using the Joinpoint Regression Program, 1975-2017 with up to five joinpoints, 2000-2017 with up to three joinpoints, all ages by race/ethnicity. National Cancer Institute. Bethesda, MD. Accessed on June 26, 2020. https://seer.cancer.gov/csr/1975_2017/, 2020.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology: Breast cancer V.4.2020. https://www.nccn.org/, 2020.
- Correa C, McGale P, Taylor C, Wang Y, et al. for the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010(41):162-77, 2010.
- Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 11:CD000563, 2013.
- Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 12(1):21-9, 2011.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 103(6):478-88, 2011.
- Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 31(32):4054-9, 2013.
- Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 32:3613-18, 2014.
- Stuart KE, Houssami N, Taylor R, Hayen A, Boyages J. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer. 15:890, 2015.
- Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 27(32):5319-24, 2009.
- Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database Syst Rev. 10:CD007847, 2012.
- Kane RL, Virnig BA, Shamliyan T, Wang SY, Tuttle TM, Wilt TJ. The impact of surgery, radiation, and systemic treatment on outcomes in patients with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010(41):130-3, 2010.
- Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 30(12):1268-73, 2012.
- Cuzick J, Sestak I, Forbes JF, et al. for the IBIS-II investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 383(9922):1041-8, 2014.
- Forbes JF, Sestak I, Howell A, et al. for the IBIS-II investigators. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. 387(10021):866-73, 2016.
- Visser LL, Groen EJ, van Leeuwen FE, Lips EH, Schmidt MK, Wesseling J. Predictors of an invasive breast cancer recurrence after DCIS: a systematic review and meta-analyses. Cancer Epidemiol Biomarkers Prev. 28(5):835-845, 2019.
- Stout NK, Cronin AM, Uno H, et al. Estrogen-receptor status and risk of contralateral breast cancer following DCIS. Breast Cancer Res Treat. 171(3):777-781, 2018.
- Miller ME, Muhsen S, Olcese C, et al. Contralateral breast cancer risk in women with ductal carcinoma in situ: is it high enough to justify bilateral mastectomy? Ann Surg Oncol. 24(10):2889-2897, 2017.
- Miller ME, Muhsen S, Zabor EC, et al. Risk of contralateral breast cancer in women with ductal carcinoma in situ associated with synchronous ipsilateral lobular carcinoma in situ. Ann Surg Oncol. 26(13):4317–4325, 2019.
- Warren LEG, Chen YH, Halasz LM, et al. Long-term outcomes of breast-conserving therapy for women with ductal carcinoma in situ. Breast Cancer Res Treat. 178(3):607-615, 2019.
- Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer. 103(9):1778-1784, 2005.
- Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 97(2):135-144, 2006.
- Van Zee KJ, White J, Morrow M, Harris JR. Chapter 23: Ductal carcinoma in situ and microinvasive carcinoma, in Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast, 5th edition. Lippincott Williams and Wilkins, 2014.
- Sanders ME, Schuyler PA, Simpson JF, Page DL, Dupont WD. Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol. 28(5):662-669, 2015.
- American Cancer Society. Breast Cancer Facts and Figures 2019-2020. Atlanta, GA: American Cancer Society, 2019.